REMATIQ raises €5.4 million to transform MedTech compliance with AI
Berlin-based REMATIQ, an AI-native platform for automated product compliance in MedTech, has raised €5.4 million in a Seed funding round to develop its AI technology, expand its engineering team, and drive growth across Europe and the U.S. The round is led by Project A Ventures, with participation from Amino Collective and HelloWorld, and complemented by […] The post REMATIQ raises €5.4 million to transform MedTech compliance with AI appeared first on EU-Startups.

Berlin-based REMATIQ, an AI-native platform for automated product compliance in MedTech, has raised €5.4 million in a Seed funding round to develop its AI technology, expand its engineering team, and drive growth across Europe and the U.S.
The round is led by Project A Ventures, with participation from Amino Collective and HelloWorld, and complemented by business angels such as SaaS founder Boris Lokschin (Spryker Systems) and industry veteran Timo Fleßner.
“Regulatory requirements shouldn’t slow down innovation – they should help accelerate it. With REMATIQ, we turn compliance from a hurdle into a competitive advantage,” says David Boutellier, Co-founder & CEO of REMATIQ. “Our goal is to bring life-saving MedTech solutions – from wound care to CT scanners – to patients faster. We’re grateful to our investors for believing in this vision and supporting us in reshaping the industry.”
REMATIQ was founded in 2023 by David Boutellier and Florian Scherer. They believe that regulatory requirements are essential—but they should never hold back innovation. With REMATIQ, they look to help MedTech companies reduce regulatory complexity without sacrificing quality or safety.
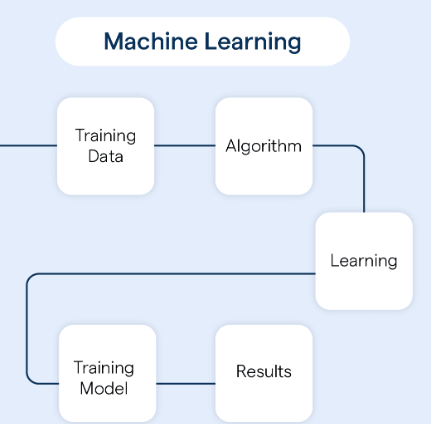
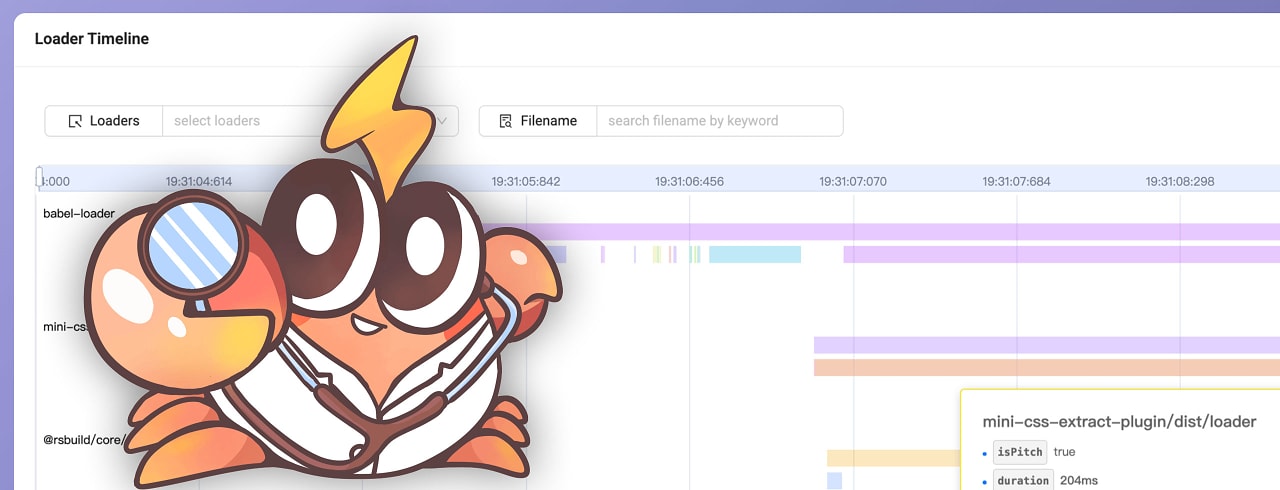
The platform uses AI to simplify compliance workflows during medical device development. It translates regulations – like MDR and FDA – into structured, actionable requirements and integrates them into companies’ existing systems. This reportedly saves up to 90% of the time typically required for regulatory documentation and coordination – freeing up engineers to focus on potentially life-changing innovations.
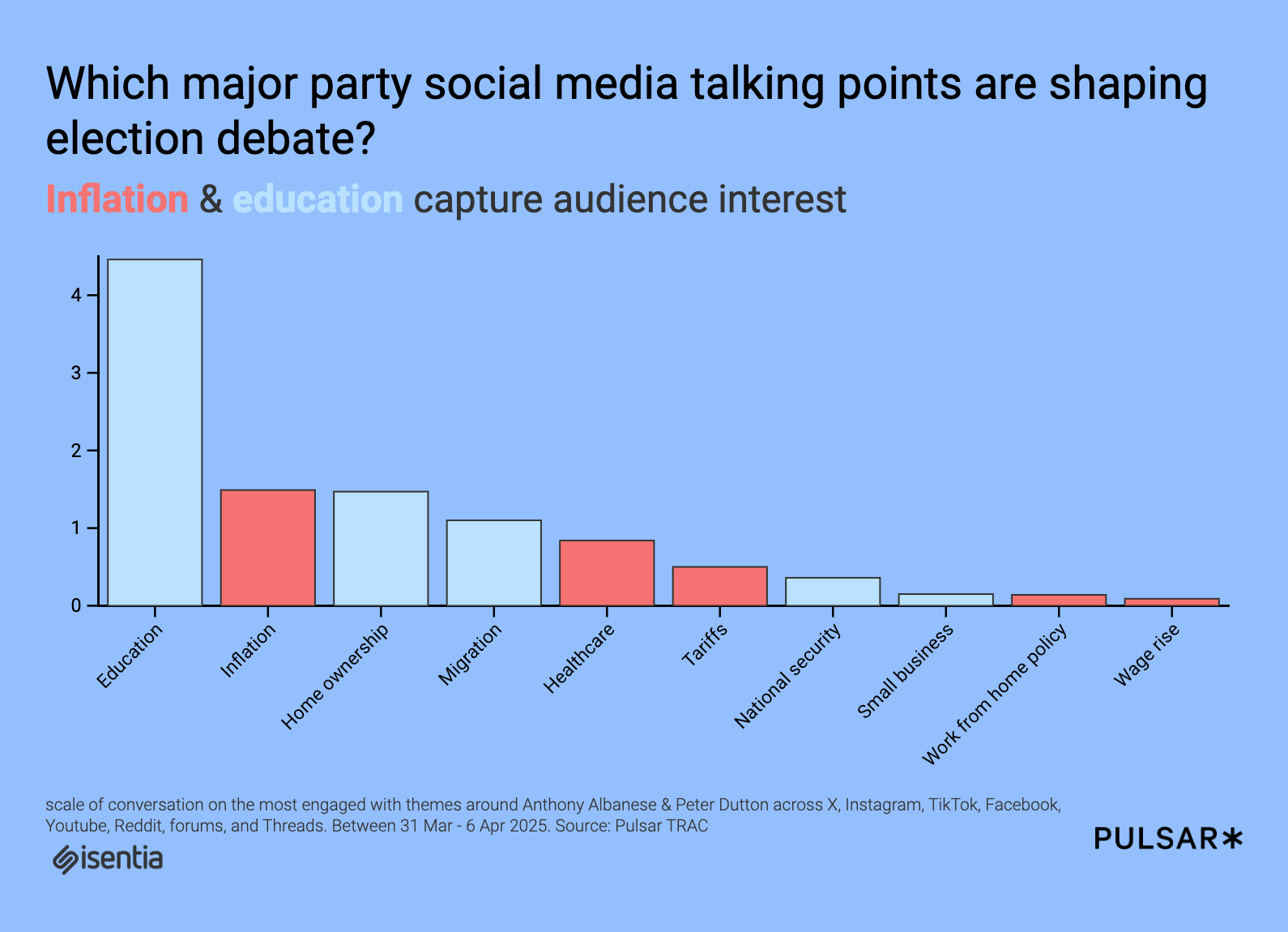
According to REMATIQ, regulatory requirements are among the biggest challenges in the MedTech industry: more than 40% of R&D teams’ time is spent on manual regulatory tasks – time that could be used for innovation.
REMATIQ’s AI-powered platform analyses global regulatory documents and provides actionable requirements. These are integrated directly into development and quality processes, reportedly reducing documentation efforts by up to 90% and significantly lowering the risk of non-compliance.
“The volume and complexity of international regulations continue to grow. We feel this every day – in development, clinical evaluation, regulatory approval, and post-market surveillance. An AI-based approach to regulatory requirements management – like the one REMATIQ offers – addresses exactly the point of highest leverage,” says Mandy Blocher, Head of PLM Digitalization & Regulatory Intelligence at the B. Braun Group.
REMATIQ plans to use the new capital to further develop its AI technology, expand its engineering team, and drive international growth across Europe and the United States.
Anton Waitz, General Partner at Project A, adds: “REMATIQ is solving a mission-critical problem. While regulatory burdens are slowing many companies down, REMATIQ offers a way to dramatically increase efficiency – without compromising on quality or safety. The team impressed us with deep industry expertise and a strong technology vision. We’re excited to support them on this journey.“
The post REMATIQ raises €5.4 million to transform MedTech compliance with AI appeared first on EU-Startups.
































































































































































![[The AI Show Episode 143]: ChatGPT Revenue Surge, New AGI Timelines, Amazon’s AI Agent, Claude for Education, Model Context Protocol & LLMs Pass the Turing Test](https://www.marketingaiinstitute.com/hubfs/ep%20143%20cover.png)






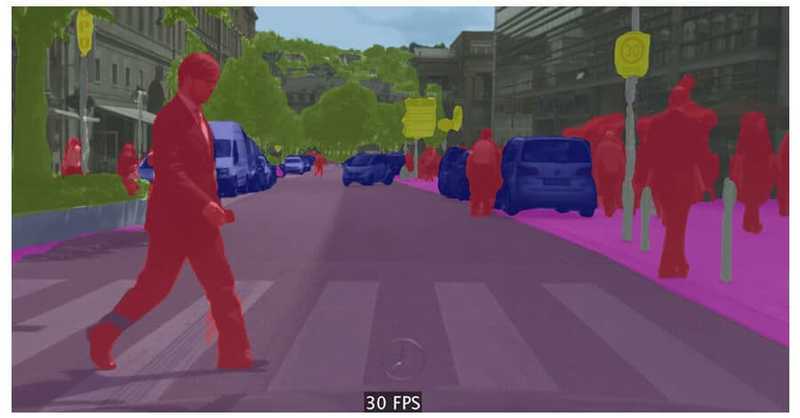
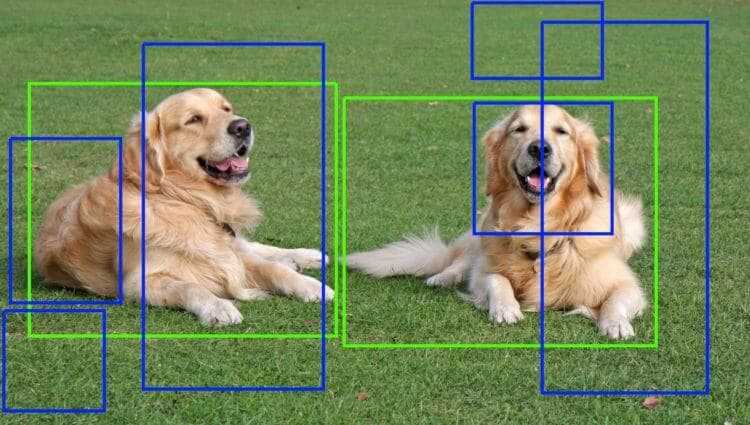

























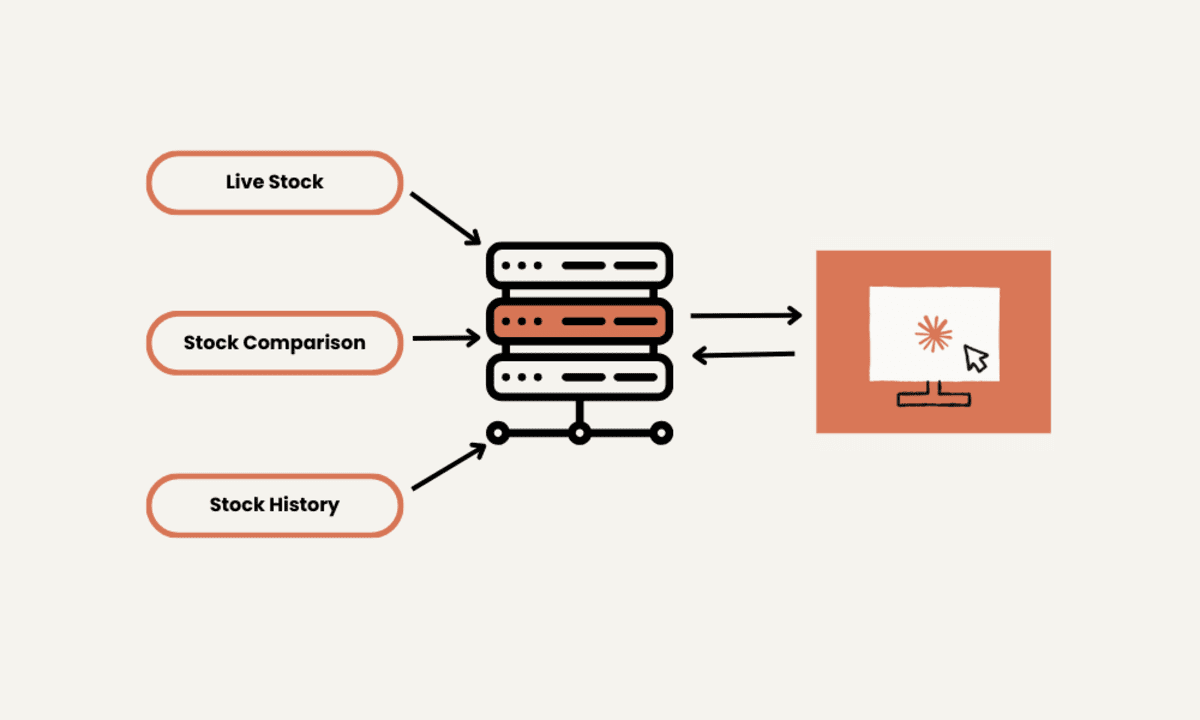
































































![From Accountant to Data Engineer with Alyson La [Podcast #168]](https://cdn.hashnode.com/res/hashnode/image/upload/v1744420903260/fae4b593-d653-41eb-b70b-031591aa2f35.png?#)






























































































.png?#)












































































































































![Apple Posts Full First Episode of 'Your Friends & Neighbors' on YouTube [Video]](https://www.iclarified.com/images/news/96990/96990/96990-640.jpg)

![Apple May Implement Global iPhone Price Increases to Mitigate Tariff Impacts [Report]](https://www.iclarified.com/images/news/96987/96987/96987-640.jpg)