Achieve Global Medical Standards with ISO 13485 Certification in Manama
In the highly regulated medical device industry, compliance and quality are not just expectations—they are non-negotiable. For organizations in Manama looking to enter or expand within the medical device market, ISO 13485 Consultants in Manama offer the expertise needed to meet international standards and ensure product safety, reliability, and regulatory readiness.
ISO 13485 is the globally recognized standard specifically designed for quality management systems (QMS) in the medical device sector. It outlines the requirements for organizations involved in the design, production, installation, and servicing of medical devices. Getting ISO 13485 Certification in Manama signals that your organization adheres to the strictest global requirements for medical device manufacturing and quality assurance.
The journey begins with a gap analysis. ISO 13485 Consultants assess your existing processes and documentation to identify areas that need alignment with the standard. This initial evaluation helps create a structured roadmap to certification, ensuring each stage is handled efficiently and compliantly.
Documentation is a critical component of ISO 13485 compliance. The standard emphasizes detailed record-keeping, traceability, risk management, and control of non-conforming products. Consultants guide you through creating and managing this documentation, training your staff, and building a quality culture that supports continuous improvement.
Internal audits and corrective actions follow, where your organization’s readiness is tested against ISO 13485’s rigorous standards. Consultants in Manama can perform mock audits, highlight non-conformities, and help implement corrective measures—ensuring a successful outcome during the final certification audit.
One of the biggest advantages of partnering with ISO 13485 Consultants in Manama is their deep understanding of both international regulations and local healthcare market dynamics. They tailor solutions specific to your operations, making the compliance journey less complex and more effective.
ISO 13485 Certification in Manama offers a range of business advantages:
-
It opens doors to international markets, including the EU and US.
-
It enhances your reputation among regulators, suppliers, and customers.
-
It improves product quality and minimizes risks.
-
It ensures faster time-to-market by reducing regulatory delays.
For startups, manufacturers, and service providers in the medical device field, this certification is not just about meeting a requirement—it's about being globally competitive, safe, and trusted. Whether you’re seeking to grow locally or export medical devices internationally, ISO 13485 Certification acts as a powerful business enabler.
In a healthcare landscape where precision and safety define success, don't leave your quality system to chance. Work with experienced professionals who can help you meet the highest standards with confidence and clarity.
Contact Us
For expert guidance get in touch with us:
Website: www.qualitcert.com
Email: contactus@qualitcert.com
Phone: +91 9686433300
#iso13485 #medicaldeviceqms #manamamedicaldevices #iso13485consultants #qualityassurance #healthtechbahrain #qms13485 #medicalcompliance #iso13485certification #bahrainhealthcare #medicaldeviceregulations #iso13485experts #qualitymanagementmanama #medtechquality #regulatorycompliance #producttraceability #iso13485implementation #qualitcertmanama #qmsconsulting #globalmedtech
































































































































































![[The AI Show Episode 148]: Microsoft’s Quiet AI Layoffs, US Copyright Office’s Bombshell AI Guidance, 2025 State of Marketing AI Report, and OpenAI Codex](https://www.marketingaiinstitute.com/hubfs/ep%20148%20cover%20%281%29.png)


![[The AI Show Episode 146]: Rise of “AI-First” Companies, AI Job Disruption, GPT-4o Update Gets Rolled Back, How Big Consulting Firms Use AI, and Meta AI App](https://www.marketingaiinstitute.com/hubfs/ep%20146%20cover.png)










































































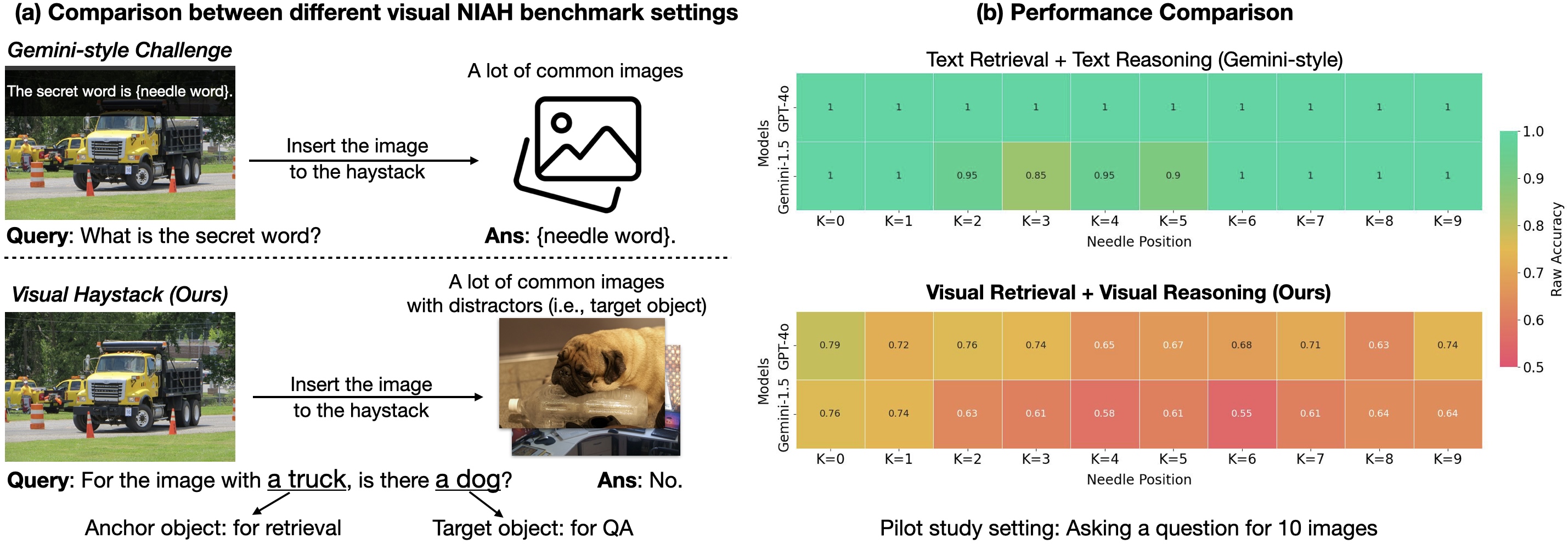














![[DEALS] Babbel Language Learning: Lifetime Subscription (All Languages) (71% off) & Other Deals Up To 98% Off – Offers End Soon!](https://www.javacodegeeks.com/wp-content/uploads/2012/12/jcg-logo.jpg)


















































.png?width=1920&height=1920&fit=bounds&quality=70&format=jpg&auto=webp#)









































![Borderlands 4 Boss Says 'A Real Fan' Will Pay $80 For Games [Update]](https://i.kinja-img.com/image/upload/c_fill,h_675,pg_1,q_80,w_1200/086e4654c281e40d12b833591d2c6fdc.jpg)














































_Constantine_Soutiaguin-Alamy.jpg?width=1280&auto=webp&quality=80&disable=upscale#)






















































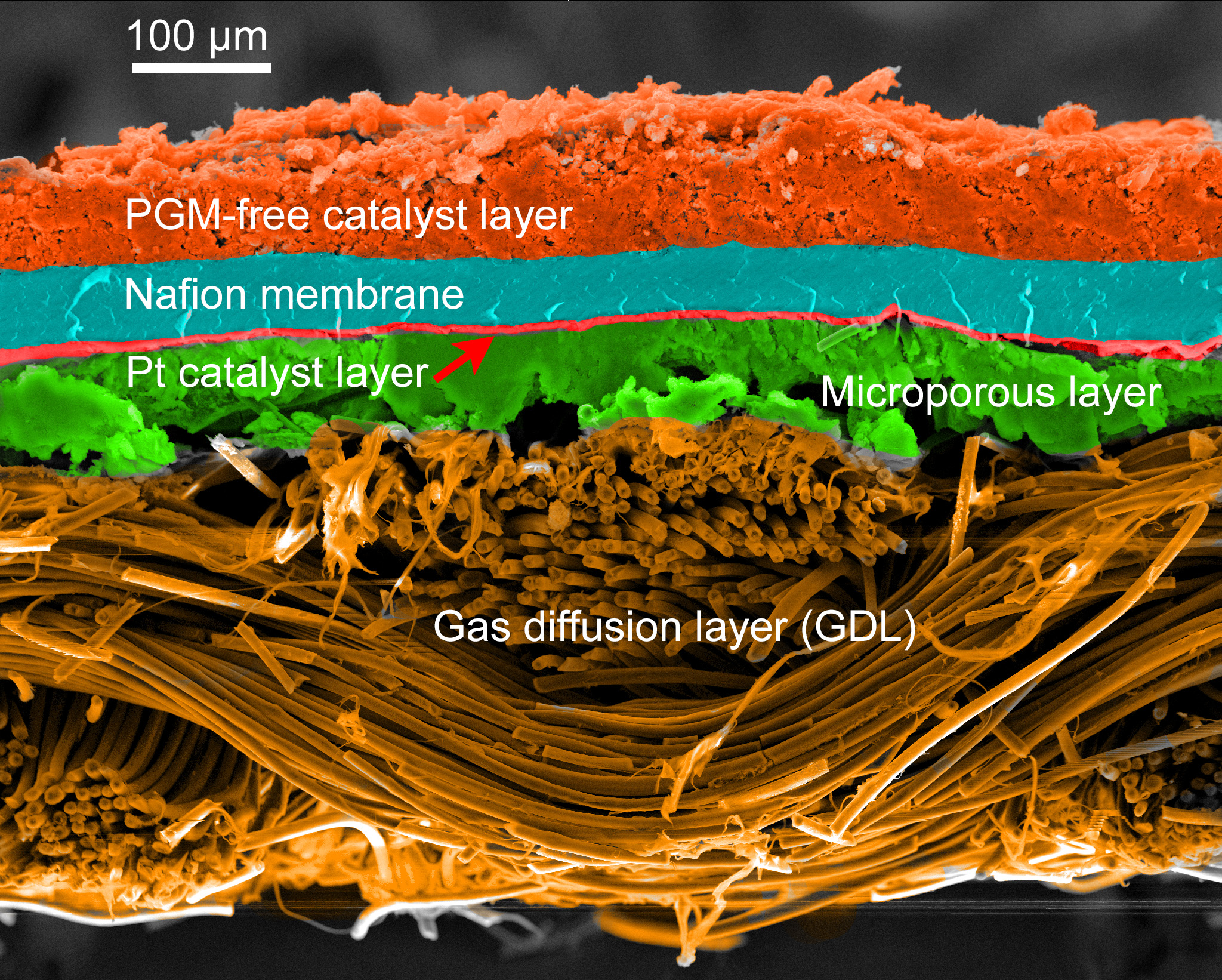




































![Nomad levels up its best-selling charger with new 100W slim adapter [Hands-on]](https://i0.wp.com/9to5mac.com/wp-content/uploads/sites/6/2025/05/100w-FI.jpg.jpg?resize=1200%2C628&quality=82&strip=all&ssl=1)



![Google just showed off Android Auto’s upcoming light theme [Gallery]](https://i0.wp.com/9to5google.com/wp-content/uploads/sites/4/2025/05/android-auto-light-theme-documentation-2.jpg?resize=1200%2C628&quality=82&strip=all&ssl=1)











![Jony Ive and OpenAI Working on AI Device With No Screen [Kuo]](https://www.iclarified.com/images/news/97401/97401/97401-640.jpg)

![Anthropic Unveils Claude 4 Models That Could Power Apple Xcode AI Assistant [Video]](https://www.iclarified.com/images/news/97407/97407/97407-640.jpg)
![Apple Leads Global Wireless Earbuds Market in Q1 2025 [Chart]](https://www.iclarified.com/images/news/97394/97394/97394-640.jpg)