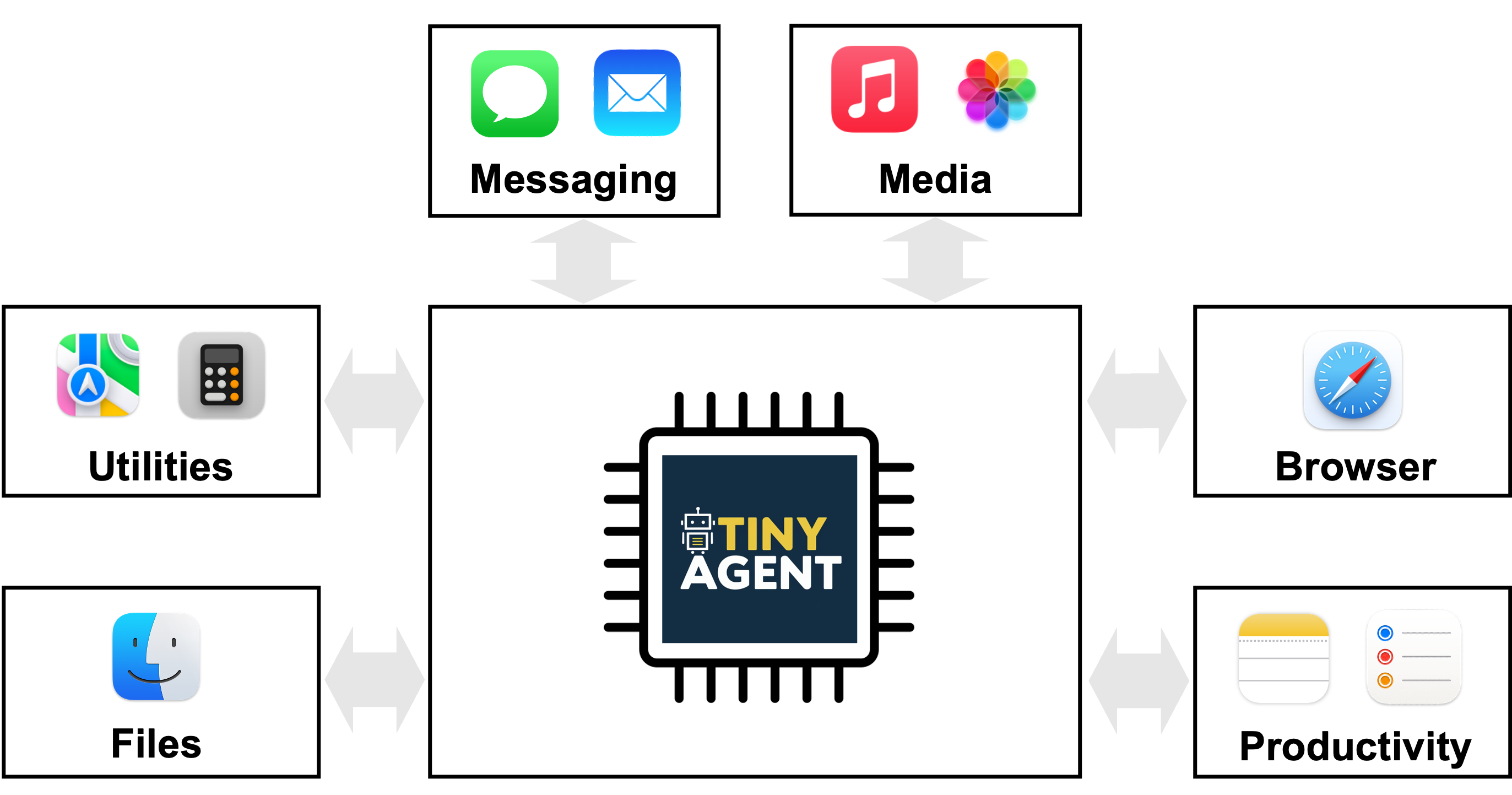
Achieve ISO 13485 Certification in Bahrain with Trusted Consulting Support
In Bahrain’s fast-evolving healthcare and medical device sector, ensuring quality, safety, and compliance isn't just good practice—it’s mandatory. For organizations involved in the design, development, production, and servicing of medical devices, achieving ISO 13485 certification is a critical step toward meeting global standards. With the support of professional ISO 13485 Consultants in Bahrain, businesses can confidently align their systems with international requirements and unlock new market opportunities.
ISO 13485 is the globally accepted standard for quality management systems specific to the medical device industry. It sets stringent criteria for ensuring consistent design, development, production, and post-market support for medical devices. For companies operating in Bahrain, compliance with ISO 13485 is increasingly being recognized as a prerequisite to doing business both locally and internationally.
Achieving ISO 13485 certification isn’t a simple checkbox process—it involves a deep integration of quality principles into every phase of the product lifecycle. This includes risk management, traceability, documentation control, supplier evaluation, and regulatory compliance. The role of skilled consultants becomes essential here. They offer expert insights, streamlined processes, and compliance strategies tailored to your business needs and regulatory environment in Bahrain.
The certification journey typically begins with a gap analysis to compare your current practices against ISO 13485 requirements. Consultants then assist in designing a roadmap to bridge these gaps. This includes documentation development, process reengineering, training, and internal audits. Their hands-on approach ensures your team not only complies with the standard but fully understands and adopts quality-first thinking.
Another key benefit of working with experienced ISO 13485 Consultants in Bahrain is their local regulatory knowledge and industry experience. They bring clarity to complex requirements and provide practical solutions that align with both ISO and National Health Regulatory Authority (NHRA) expectations.
Once the system is fully implemented and refined, your business will be ready for the final certification audit. Consultants often conduct mock audits and provide detailed feedback to ensure you're fully prepared. The result is a robust quality management system that supports regulatory approvals, product safety, and patient trust.
For businesses in Bahrain aiming to export medical devices or serve international markets, ISO 13485 certification offers a strong competitive edge. It opens doors to global partnerships, increases stakeholder confidence, and reduces the risk of costly compliance failures.
In a highly regulated industry like medical devices, there is no room for shortcuts. Choosing a consulting partner who understands the technical and regulatory landscape is vital. Let ISO 13485 Certification in Bahrain be your stepping stone to achieving excellence, compliance, and global recognition.
Contact Us
For expert guidance get in touch with us:
Website: www.qualitcert.com
Email: contactus@qualitcert.com
Phone: +91 9686433300
#iso13485bahrain #medicaldevicequality #iso13485certification #iso13485consultants #bahrainhealthcare #qualitymanagement #iso13485implementation #qmsmedicaldevices #iso13485experts #iso13485services #qualitcertbahrain #iso13485standard #medtechbahrain #iso13485training #regulatorycompliance #iso13485audit #bahrainqms #healthcarestandards #medicaldevicecompliance #iso13485benefits


































































































































































![[The AI Show Episode 148]: Microsoft’s Quiet AI Layoffs, US Copyright Office’s Bombshell AI Guidance, 2025 State of Marketing AI Report, and OpenAI Codex](https://www.marketingaiinstitute.com/hubfs/ep%20148%20cover%20%281%29.png)


![[The AI Show Episode 146]: Rise of “AI-First” Companies, AI Job Disruption, GPT-4o Update Gets Rolled Back, How Big Consulting Firms Use AI, and Meta AI App](https://www.marketingaiinstitute.com/hubfs/ep%20146%20cover.png)
























































































































































































































_Prostock-studio_Alamy.jpg?width=1280&auto=webp&quality=80&disable=upscale#)








































































































































![Mobile Legends: Bang Bang [MLBB] Free Redeem Codes May 2025](https://www.talkandroid.com/wp-content/uploads/2024/07/Screenshot_20240704-093036_Mobile-Legends-Bang-Bang.jpg)