How Serigen Is Pioneering Regenerative Medtech in India
Discover how Serigen is redefining tissue repair with silk-based innovations that are affordable, effective, and globally scalable.


In India's vast landscape of medtech innovation, few startups have woven together tradition and technology quite like Serigen Mediproducts. Drawing from the country’s rich sericulture ecosystem, Serigen is crafting a new narrative in tissue regeneration, one built not on synthetic polymers or expensive imports, but on the untapped biomedical power of silk.
The Silk Road to Innovation
Founded in 2015 in Pune by Dr. Anuya Nisal, along with co-founders Dr. Swati Shukla and Dr. Premnath Venugopalan, Serigen emerged from over a decade of research at CSIR-National Chemical Laboratory. Their pivotal discovery: silk protein, a sustainable and indigenous material, holds immense potential in healing bones, wounds, and soft tissue.
"India is a global silk producer, yet its application in healthcare remains largely unexplored. We wanted to change that,” says Dr. Nisal.
With a mission to make world-class, clinician-trusted tissue repair products, Serigen blends materials science, polymer chemistry, and clinical rigour to create innovations that are not only effective but also affordable and scalable.
Clinical Science with Social Impact
Serigen's flagship products harness silk protein's biocompatibility to outperform traditional wound and bone care treatments:
- Seriderm: Accelerates wound healing, used in over 300 patients.
- Serioss: A global first, clinically validated for bone regeneration.
- Serimat: Targets soft tissue restoration post-surgery or trauma.
These products address pressing gaps in India’s healthcare system, offering high-performance alternatives to synthetic grafts at an affordable cost. All are manufactured in Serigen’s ISO 13485-certified facility, backed by over 25 regulatory approvals and multiple global patents.
Building a Medtech Ecosystem from Pune
Incubated at Venture Centre, Serigen's team is structured across R&D, clinical affairs, manufacturing, regulatory, and sales. Its founders combine academic prowess with industry experience from GE Plastics and CSIR, bringing deep expertise to every product lifecycle.
"We value interdisciplinary talent and a passion for real-world problem-solving," says Dr. Shukla. Their approach has attracted key institutional buyers, KOL endorsements, and B2B partnerships, alongside consumer-facing sales on platforms like Tata 1mg.
Market Opportunity and Global Ambition
With the global tissue regeneration market valued at over $19 billion, Serigen Mediproducts sees significant growth potential both within India and internationally. Its silk-based wound care products are already being used in leading Indian hospitals, and the company is now preparing to expand into Southeast Asia, Europe, and the United States.
Among its recent milestones, Serigen has successfully treated over 300 wound care patients, conducted multiple clinical trials focused on bone regeneration, and raised INR 22.6 crore through angel, seed, grant and pre-Series A funding rounds.
The startup’s scientific achievements have been recognised through prestigious awards from the Government of India, the Royal Academy of Engineering (UK), and the Orthopaedic Research Society (USA).
Challenges and Takeaways
The journey hasn’t been without friction. Long clinical validation cycles and scepticism around new biomaterials remain barriers. But Serigen’s strategy is grounded in science and persistence.
"Deeptech needs patience, but its rewards are transformative," says Dr. Nisal.
Looking Ahead: From India to the World
Serigen plans to launch Serioss nationally by end-2025 and scale Seriderm into global markets pending US FDA and EU approvals. Its long-term goal: to become the global leader in silk-based regenerative medicine.
As the startup continues to blend India's scientific acumen with its sericultural heritage, Serigen is proving that medical innovation can be both local in origin and global in ambition.





























































































































































![[The AI Show Episode 146]: Rise of “AI-First” Companies, AI Job Disruption, GPT-4o Update Gets Rolled Back, How Big Consulting Firms Use AI, and Meta AI App](https://www.marketingaiinstitute.com/hubfs/ep%20146%20cover.png)






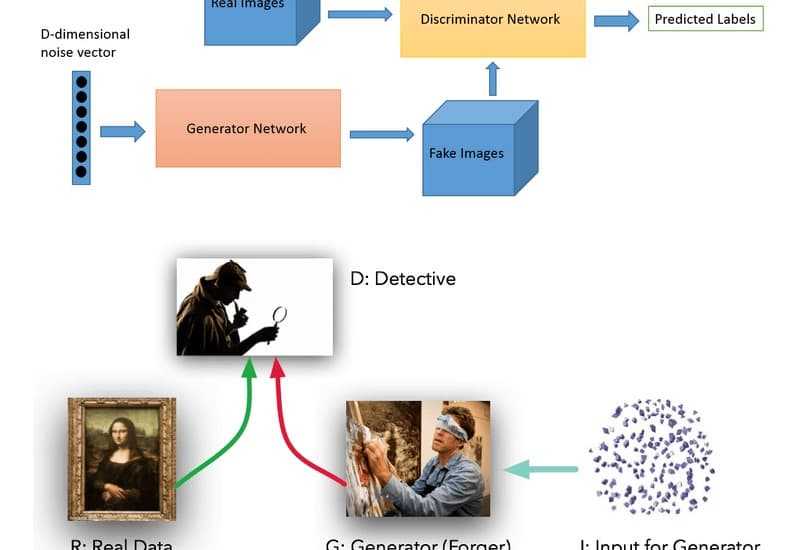
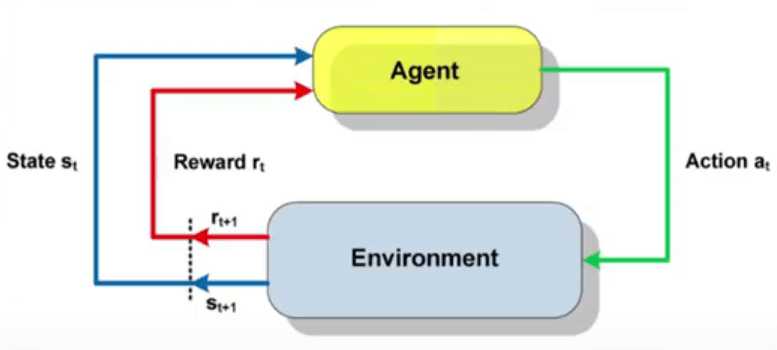
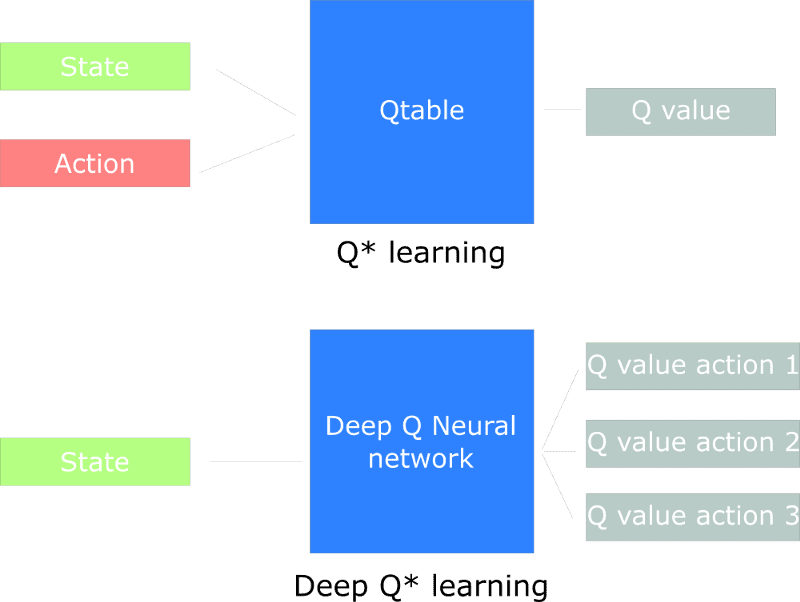
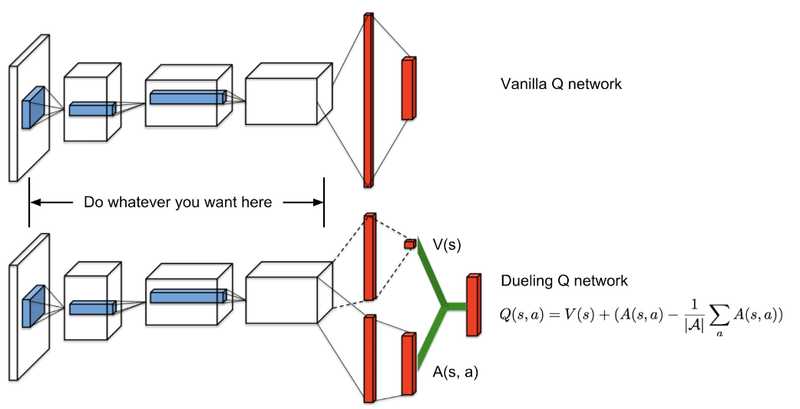




















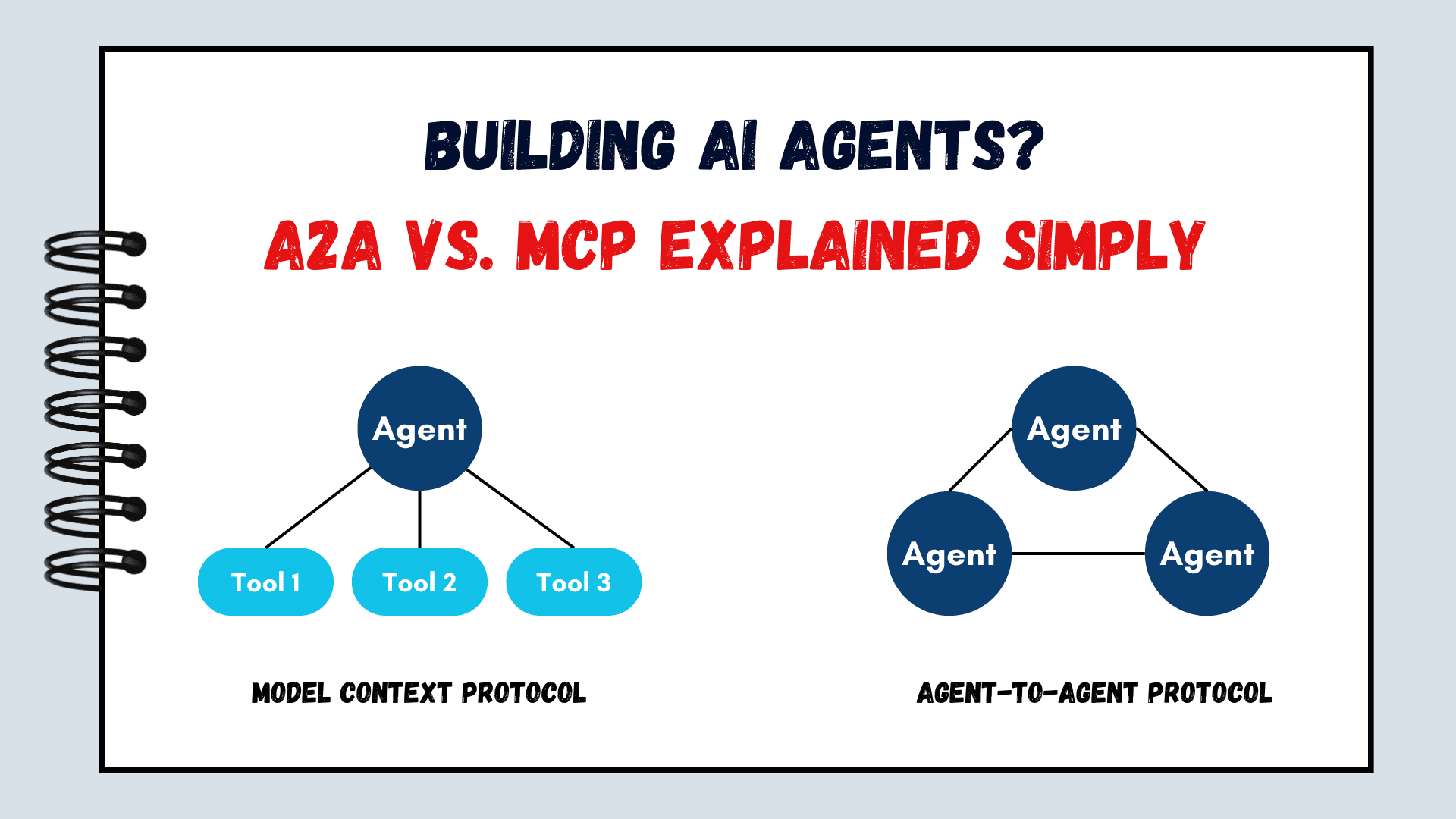
































































![How to make Developer Friends When You Don't Live in Silicon Valley, with Iraqi Engineer Code;Life [Podcast #172]](https://cdn.hashnode.com/res/hashnode/image/upload/v1747360508340/f07040cd-3eeb-443c-b4fb-370f6a4a14da.png?#)











































































































.png?width=1920&height=1920&fit=bounds&quality=70&format=jpg&auto=webp#)





















![[Virtual Event] Strategic Security for the Modern Enterprise](https://eu-images.contentstack.com/v3/assets/blt6d90778a997de1cd/blt55e4e7e277520090/653a745a0e92cc040a3e9d7e/Dark_Reading_Logo_VirtualEvent_4C.png?width=1280&auto=webp&quality=80&disable=upscale#)













































































-xl-(1)-xl-xl.jpg)










![How to upgrade the M4 Mac mini SSD and save hundreds [Video]](https://i0.wp.com/9to5mac.com/wp-content/uploads/sites/6/2025/05/M4-Mac-mini-SSD-Upgrade-Tutorial-2TB.jpg?resize=1200%2C628&quality=82&strip=all&ssl=1)
![‘Apple in China’ book argues that the iPhone could be killed overnight [Updated]](https://i0.wp.com/9to5mac.com/wp-content/uploads/sites/6/2025/05/Apple-in-China-review.jpg?resize=1200%2C628&quality=82&strip=all&ssl=1)













![iPhone 17 Air Could Get a Boost From TDK's New Silicon Battery Tech [Report]](https://www.iclarified.com/images/news/97344/97344/97344-640.jpg)
![Vision Pro Owners Say They Regret $3,500 Purchase [WSJ]](https://www.iclarified.com/images/news/97347/97347/97347-640.jpg)
![Apple Showcases 'Magnifier on Mac' and 'Music Haptics' Accessibility Features [Video]](https://www.iclarified.com/images/news/97343/97343/97343-640.jpg)
![Sony WH-1000XM6 Unveiled With Smarter Noise Canceling and Studio-Tuned Sound [Video]](https://www.iclarified.com/images/news/97341/97341/97341-640.jpg)





































![Apple Stops Signing iPadOS 17.7.7 After Reports of App Login Issues [Updated]](https://images.macrumors.com/t/DoYicdwGvOHw-VKkuNvoxYs3pfo=/1920x/article-new/2023/06/ipados-17.jpg)



























