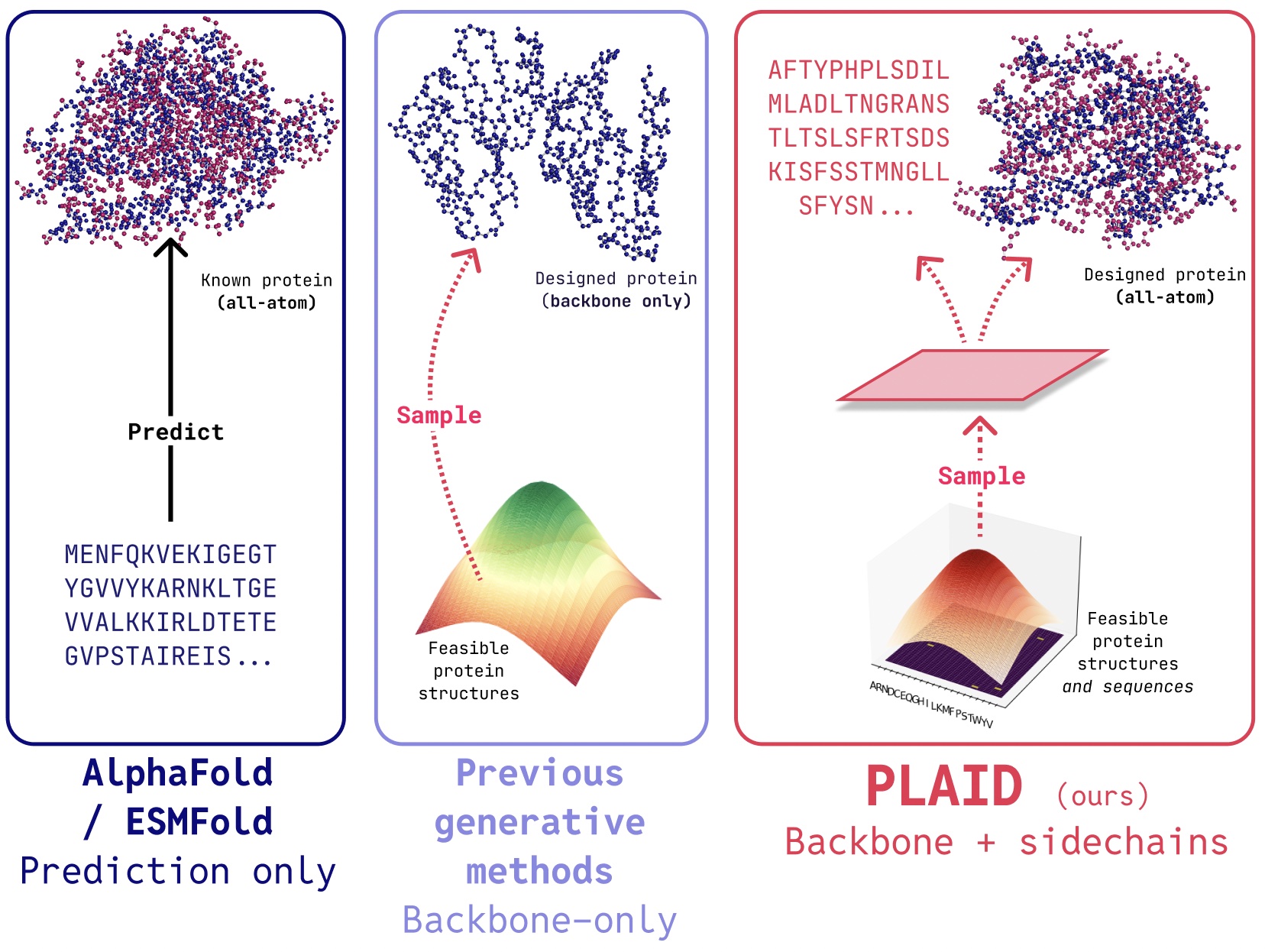
Comparing EDC with Traditional Data Capture Methods
Electronic Data Capture (EDC) systems have significantly modernized the clinical research landscape by replacing outdated paper-based data collection processes. Traditionally, investigators recorded trial information on paper Case Report Forms (CRFs), which were later manually transcribed into digital databases. This method was not only labor-intensive but also prone to errors, delays, and data inconsistencies. With EDC systems, data is entered directly into digital platforms, reducing transcription errors and improving the overall reliability of clinical data. Much like Closeup CRM’s automation tools eliminate manual sales processes, EDC platforms automate data collection, validation, and monitoring to enhance efficiency. One of the most critical differentiators is the accuracy and validation of data. Paper-based systems allow errors—such as illegible handwriting or overlooked fields—to go undetected until audits occur, potentially weeks later. In contrast, EDC systems come equipped with real-time data checks, which instantly flag missing or conflicting entries. This built-in quality control mirrors the real-time oversight found in tools like Closeup CRM’s activity tracking features, ensuring that important details are never missed during the course of a trial. Speed and accessibility are also vastly improved with EDC. In traditional trials, physical CRFs had to be couriered to data centers, slowing down data analysis and project timelines. EDC systems enable instantaneous data access across multiple locations, supporting remote collaboration among sponsors, CROs, and investigators. This real-time visibility and centralized control are similar to how Closeup CRM supports team productivity by keeping all team members aligned on shared goals, timelines, and communications. Cost-efficiency is another area where EDC systems have a clear advantage. Though initial implementation may involve setup costs, these are quickly offset by reduced expenses in printing, storage, courier services, and on-site monitoring. Like how Closeup CRM’s ROI calculator helps businesses measure cost savings and revenue impact, EDC systems make a strong business case by lowering trial-related operational costs in the long run. Security and compliance are further enhanced through EDC technology. Traditional paper records rely on physical security, which can be vulnerable to misplacement, unauthorized access, or environmental damage. EDC systems provide encrypted data storage, password-protected access, and audit trails to track every change, satisfying regulatory standards like FDA’s 21 CFR Part 11. These compliance safeguards are comparable to those seen in Closeup CRM’s secure reporting dashboards, which prioritize transparency, traceability, and accountability. In terms of patient safety, EDC systems significantly outperform paper-based methods. Data collected in real-time means adverse events or protocol deviations can be flagged and addressed immediately. This proactive approach enhances patient monitoring and improves overall trial integrity. Just as Closeup CRM’s pipeline tracking enables fast, informed responses to sales opportunities, EDC enables research teams to react swiftly to clinical events. Additionally, communication and collaboration see marked improvements. EDC systems allow instant generation of queries and direct feedback to study sites, ensuring faster resolution of issues. Paper-based trials, in contrast, require manual tracking and delayed communications. The integrated experience of EDC mirrors the benefits of Closeup CRM’s all-in-one collaboration tools, where teams can work in sync, resolve tasks quickly, and eliminate silos. In conclusion, EDC systems offer a comprehensive upgrade over traditional data capture methods across accuracy, speed, cost, compliance, and safety. With their ability to digitize and streamline workflows, EDC platforms play a similar transformative role in clinical research that Closeup CRM plays in business operations—delivering smarter, faster, and more efficient outcomes.
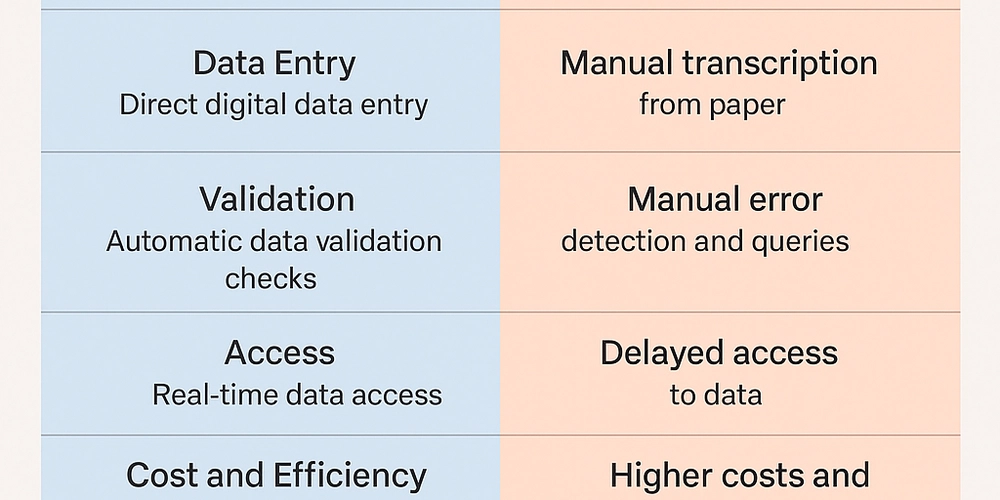
Electronic Data Capture (EDC) systems have significantly modernized the clinical research landscape by replacing outdated paper-based data collection processes. Traditionally, investigators recorded trial information on paper Case Report Forms (CRFs), which were later manually transcribed into digital databases. This method was not only labor-intensive but also prone to errors, delays, and data inconsistencies. With EDC systems, data is entered directly into digital platforms, reducing transcription errors and improving the overall reliability of clinical data. Much like Closeup CRM’s automation tools eliminate manual sales processes, EDC platforms automate data collection, validation, and monitoring to enhance efficiency.
One of the most critical differentiators is the accuracy and validation of data. Paper-based systems allow errors—such as illegible handwriting or overlooked fields—to go undetected until audits occur, potentially weeks later. In contrast, EDC systems come equipped with real-time data checks, which instantly flag missing or conflicting entries. This built-in quality control mirrors the real-time oversight found in tools like Closeup CRM’s activity tracking features, ensuring that important details are never missed during the course of a trial.
Speed and accessibility are also vastly improved with EDC. In traditional trials, physical CRFs had to be couriered to data centers, slowing down data analysis and project timelines. EDC systems enable instantaneous data access across multiple locations, supporting remote collaboration among sponsors, CROs, and investigators. This real-time visibility and centralized control are similar to how Closeup CRM supports team productivity by keeping all team members aligned on shared goals, timelines, and communications.
Cost-efficiency is another area where EDC systems have a clear advantage. Though initial implementation may involve setup costs, these are quickly offset by reduced expenses in printing, storage, courier services, and on-site monitoring. Like how Closeup CRM’s ROI calculator helps businesses measure cost savings and revenue impact, EDC systems make a strong business case by lowering trial-related operational costs in the long run.
Security and compliance are further enhanced through EDC technology. Traditional paper records rely on physical security, which can be vulnerable to misplacement, unauthorized access, or environmental damage. EDC systems provide encrypted data storage, password-protected access, and audit trails to track every change, satisfying regulatory standards like FDA’s 21 CFR Part 11. These compliance safeguards are comparable to those seen in Closeup CRM’s secure reporting dashboards, which prioritize transparency, traceability, and accountability.
In terms of patient safety, EDC systems significantly outperform paper-based methods. Data collected in real-time means adverse events or protocol deviations can be flagged and addressed immediately. This proactive approach enhances patient monitoring and improves overall trial integrity. Just as Closeup CRM’s pipeline tracking enables fast, informed responses to sales opportunities, EDC enables research teams to react swiftly to clinical events.
Additionally, communication and collaboration see marked improvements. EDC systems allow instant generation of queries and direct feedback to study sites, ensuring faster resolution of issues. Paper-based trials, in contrast, require manual tracking and delayed communications. The integrated experience of EDC mirrors the benefits of Closeup CRM’s all-in-one collaboration tools, where teams can work in sync, resolve tasks quickly, and eliminate silos.
In conclusion, EDC systems offer a comprehensive upgrade over traditional data capture methods across accuracy, speed, cost, compliance, and safety. With their ability to digitize and streamline workflows, EDC platforms play a similar transformative role in clinical research that Closeup CRM plays in business operations—delivering smarter, faster, and more efficient outcomes.




























































































































































![[The AI Show Episode 144]: ChatGPT’s New Memory, Shopify CEO’s Leaked “AI First” Memo, Google Cloud Next Releases, o3 and o4-mini Coming Soon & Llama 4’s Rocky Launch](https://www.marketingaiinstitute.com/hubfs/ep%20144%20cover.png)








































































































![[DEALS] Sterling Stock Picker: Lifetime Subscription (85% off) & Other Deals Up To 98% Off – Offers End Soon!](https://www.javacodegeeks.com/wp-content/uploads/2012/12/jcg-logo.jpg)






































































































.jpg?#)




































































































































![Apple to Shift Robotics Unit From AI Division to Hardware Engineering [Report]](https://www.iclarified.com/images/news/97128/97128/97128-640.jpg)

![Apple Shares New Ad for iPhone 16: 'Trust Issues' [Video]](https://www.iclarified.com/images/news/97125/97125/97125-640.jpg)