mRNA and saRNA Vaccine Persistence: A Hidden Dialogue with Estrogen Signaling?
Introduction Synthetic RNA technologies, such as mRNA and self-amplifying RNA (saRNA), have rapidly gained attention as key platforms for vaccine development. While these technologies have proven effective in infectious disease control, questions are emerging about the long-term biological presence of these RNAs and their potential interactions with host cellular pathways. This post explores the possibility of crosstalk between RNA vaccine persistence and estrogen signaling—an area still underexplored but potentially significant for sex-specific responses and endocrine-sensitive populations. Background: What Do We Know About RNA Vaccine Persistence? Initial clinical assumptions surrounding mRNA and saRNA vaccines emphasized their transient nature—designed to degrade rapidly after eliciting an immune response. However, recent studies have challenged this view. Evidence from biodistribution studies shows that synthetic RNAs, encapsulated in lipid nanoparticles, can persist in lymph nodes, liver, and muscle tissues for weeks, and in some cases, months. Additionally, research such as the 2022 Aldén et al. study has raised the possibility of reverse transcription of vaccine mRNA into host DNA, at least in hepatic cell lines. While the clinical significance remains debated, these findings suggest that synthetic RNA may have more complex biological interactions than originally anticipated. This persistence and potential integration raise new questions, particularly when considering RNA stability in hormonally active tissues. Estrogen Signaling and the Unexpected Crosstalk Emerging studies suggest that estrogen receptors—particularly ERα—may play a role in modulating immune responses to synthetic RNA. These receptors are expressed in various cell types, including immune cells and hepatocytes, which are also implicated in RNA vaccine biodistribution and reverse transcription events. Notably, estrogen is known to influence the expression of endogenous retroelements and the regulation of APOBEC enzymes, both of which intersect with RNA editing and potential integration pathways. This opens the door to hypotheses where estrogen signaling could amplify or mitigate the biological activity of synthetic RNA within specific tissue environments. This crosstalk is particularly relevant in populations with fluctuating hormone levels, such as women of reproductive age, postmenopausal individuals, and those undergoing hormone therapy. Sex-specific responses to RNA-based therapeutics may not be solely immunological but also endocrinological in nature. Implications for Endocrine Function and Personalized Medicine The potential interaction between mRNA and saRNA vaccines and estrogen signaling has far-reaching implications for endocrine function. If these vaccines indeed persist in hormonally active tissues and interact with estrogen receptors, this could influence a wide array of physiological processes, including metabolic regulation, immune responses, and tissue repair. For personalized medicine, this discovery opens the door to more tailored vaccination strategies. By considering an individual's hormonal profile—whether related to sex, age, or hormone therapies—clinicians might be able to better predict responses to RNA-based vaccines. This is particularly important in populations such as postmenopausal women, those undergoing hormone replacement therapy (HRT), or individuals with hormone-sensitive conditions. Understanding how estrogen and RNA vaccines interact could also help in designing vaccines that are more effective for certain subgroups or even in adjusting vaccine formulations to reduce adverse effects in hormonally sensitive populations. Ultimately, this approach could lead to more precise and individualized vaccine protocols, improving both the safety and efficacy of these therapies. Conclusion: What Questions Should We Be Asking? As we explore the intersection of synthetic RNA vaccines and estrogen signaling, several important questions remain unanswered. First and foremost, how significant is the persistence of RNA in hormonally active tissues over the long term, and what are the physiological implications of this persistence? While we have seen evidence suggesting RNA’s ability to linger in the body, especially in tissues like the liver and lymph nodes, we still lack comprehensive studies on its long-term effects on hormone-sensitive tissues. Another critical question is whether estrogen’s modulation of RNA vaccine responses could be leveraged to improve vaccine efficacy or safety in different populations. Given the known influence of estrogen on immune function, understanding its role could be key to optimizing vaccine strategies for both women and men, particularly those with hormone-related conditions. Moreover, the potential for personalized RNA vaccine therapies based on hormonal profiles presents a new frontier in precision medicine. How can
Introduction
Synthetic RNA technologies, such as mRNA and self-amplifying RNA (saRNA), have rapidly gained attention as key platforms for vaccine development. While these technologies have proven effective in infectious disease control, questions are emerging about the long-term biological presence of these RNAs and their potential interactions with host cellular pathways. This post explores the possibility of crosstalk between RNA vaccine persistence and estrogen signaling—an area still
underexplored but potentially significant for sex-specific responses and endocrine-sensitive populations.
Background: What Do We Know About RNA Vaccine Persistence?
Initial clinical assumptions surrounding mRNA and saRNA vaccines emphasized their transient nature—designed to degrade rapidly after eliciting an immune response. However, recent studies have challenged this view. Evidence from biodistribution studies shows that synthetic RNAs, encapsulated in lipid nanoparticles, can persist in lymph nodes, liver, and muscle tissues for weeks, and in some cases, months.
Additionally, research such as the 2022 Aldén et al. study has raised the possibility of reverse transcription of vaccine mRNA into host DNA, at least in hepatic cell lines. While the clinical significance remains debated, these findings suggest that synthetic RNA may have more complex biological interactions than originally anticipated.
This persistence and potential integration raise new questions, particularly when considering RNA stability in hormonally active tissues.
Estrogen Signaling and the Unexpected Crosstalk
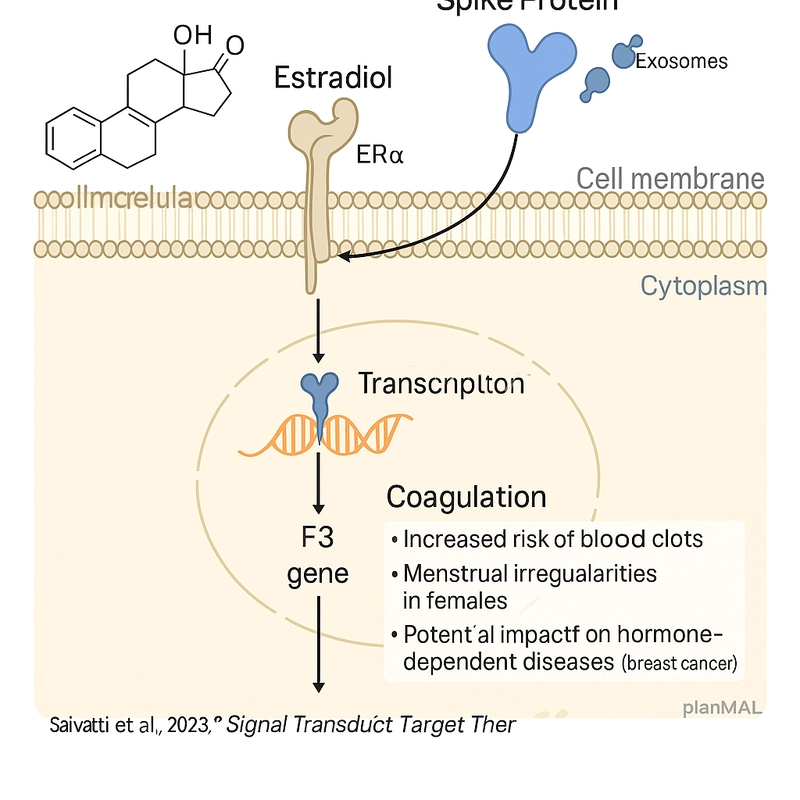
Emerging studies suggest that estrogen receptors—particularly ERα—may play a role in modulating immune responses to synthetic RNA. These receptors are expressed in various cell types, including immune cells and hepatocytes, which are also implicated in RNA vaccine biodistribution and reverse transcription events.
Notably, estrogen is known to influence the expression of endogenous retroelements and the regulation of APOBEC enzymes, both of which intersect with RNA editing and potential integration pathways. This opens the door to hypotheses where estrogen signaling could amplify or mitigate the biological activity of synthetic RNA within specific tissue environments.
This crosstalk is particularly relevant in populations with fluctuating hormone levels, such as women of reproductive age, postmenopausal individuals, and those undergoing hormone therapy. Sex-specific responses to RNA-based therapeutics may not be solely immunological but also endocrinological in nature.
Implications for Endocrine Function and Personalized Medicine
The potential interaction between mRNA and saRNA vaccines and estrogen signaling has far-reaching implications for endocrine function. If these vaccines indeed persist in hormonally active tissues and interact with estrogen receptors, this could influence a wide array of physiological processes, including metabolic regulation, immune responses, and tissue repair.
For personalized medicine, this discovery opens the door to more tailored vaccination strategies. By considering an individual's hormonal profile—whether related to sex, age, or hormone therapies—clinicians might be able to better predict responses to RNA-based vaccines. This is particularly important in populations such as postmenopausal women, those undergoing hormone replacement therapy (HRT), or individuals with hormone-sensitive conditions.
Understanding how estrogen and RNA vaccines interact could also help in designing vaccines that are more effective for certain subgroups or even in adjusting vaccine formulations to reduce adverse effects in hormonally sensitive populations. Ultimately, this approach could lead to more precise and individualized vaccine protocols, improving both the safety and efficacy of these therapies.
Conclusion: What Questions Should We Be Asking?
As we explore the intersection of synthetic RNA vaccines and estrogen signaling, several important questions remain unanswered. First and foremost, how significant is the persistence of RNA in hormonally active tissues over the long term, and what are the physiological implications of this persistence? While we have seen evidence suggesting RNA’s ability to linger in the body, especially in tissues like the liver and lymph nodes, we still lack comprehensive studies on its long-term effects on hormone-sensitive tissues.
Another critical question is whether estrogen’s modulation of RNA vaccine responses could be leveraged to improve vaccine efficacy or safety in different populations. Given the known influence of estrogen on immune function, understanding its role could be key to optimizing vaccine strategies for both women and men, particularly those with hormone-related conditions.
Moreover, the potential for personalized RNA vaccine therapies based on hormonal profiles presents a new frontier in precision medicine. How can clinicians best integrate hormonal considerations into vaccine development, and what biomarkers would be most useful for predicting individual responses? These questions underscore the need for more research to fully understand the dynamic interactions between RNA vaccines and endocrine pathways.
Ultimately, the emerging science surrounding RNA vaccines and their hormonal interactions highlights the complexity of human biology and the importance of considering sex and hormonal factors in the development of future therapeutics.

































































































































































![[The AI Show Episode 144]: ChatGPT’s New Memory, Shopify CEO’s Leaked “AI First” Memo, Google Cloud Next Releases, o3 and o4-mini Coming Soon & Llama 4’s Rocky Launch](https://www.marketingaiinstitute.com/hubfs/ep%20144%20cover.png)


































































































![[DEALS] The All-in-One Microsoft Office Pro 2019 for Windows: Lifetime License + Windows 11 Pro Bundle (89% off) & Other Deals Up To 98% Off](https://www.javacodegeeks.com/wp-content/uploads/2012/12/jcg-logo.jpg)









































































































































_Andreas_Prott_Alamy.jpg?width=1280&auto=webp&quality=80&disable=upscale#)

























































































![What features do you get with Gemini Advanced? [April 2025]](https://i0.wp.com/9to5google.com/wp-content/uploads/sites/4/2024/02/gemini-advanced-cover.jpg?resize=1200%2C628&quality=82&strip=all&ssl=1)












![Apple Shares Official Trailer for 'Long Way Home' Starring Ewan McGregor and Charley Boorman [Video]](https://www.iclarified.com/images/news/97069/97069/97069-640.jpg)
![Apple Watch Series 10 Back On Sale for $299! [Lowest Price Ever]](https://www.iclarified.com/images/news/96657/96657/96657-640.jpg)
![EU Postpones Apple App Store Fines Amid Tariff Negotiations [Report]](https://www.iclarified.com/images/news/97068/97068/97068-640.jpg)